5th Generation Universal Strengthened Chimeric Antigen Receptor T-Cell Immunotherapy
New Generation of CAR-T:
- Integration of suicide gene, to limit the risk of unwanted result caused by long term stay of genetically modified T cell in the system.
- Precision regulation of cytokine release and activation to avoid “cytokine storm”
- Universal CAR-T to overcome the the shortcoming of common CAR:
- highly individualized.
- unsuitable for cGMP management.
- high cost.
poor condition of T cells from the patient may not fit for genetic maneuvers.
Major Breakthroughs in USCAR-T Technology
A. Non Viral Trans-infection
- One of severe side effect of CAR-T therapy, Cerebral edema, which may be contributed by vial construct or viral preparation method. Non-viral trans-infection method can avoid this risk.
- Pinpoint insertion to avoid random viral insertion which may cause off-target effect.
- Variety of non-viral methods can be selected according to construct.
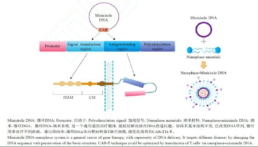
B. Universal CAR with Immune Knockout T Cell
- Low cell count and vitality of patient’s autologous T cells are often with function damage or loss, may not ideal for genetic maneuver and ex vivo expansion.
- Individualized therapy is highly expensive. The time-consuming procedure may delay treatment.
- Lab scaled preparation can hardly satisfy the needs of industrial scaled, ready-made cell manufacture.
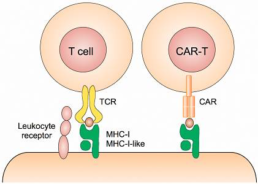
C. Inserting“Suicidal Gene”and“Molecular Switch ”
- To resolve the problems of:
- Cytokine Release Syndrome
(CRS) or “cytokine storm”
- Potential risk from the long
term stay of transgenic T cell’s
- Cancer risk from the mutation of inserted gene
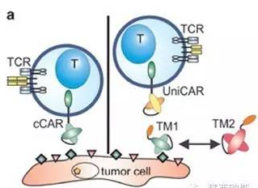
Comparison of Old and New CAR-T Technology
I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
USCAR-T non-viral method with same level of trans-infection rate
- Lower cost at least 80%
- The whole procedure shortens 9 months of treatment time
USCAR-T each treatment only cost $4000 according to Cellectis,which is 1/100 of Norvatis’CD19 (Kymriah™)procedure。
USCAR-T deactivates immune inhibitory genes like TGF-beta receptor,PD1,destroys the micro environment of solid tumors so to strengthen T cell function in treating liver matastases of colon cancer and pancreatic cancer or other solid tumor. The market is estimated at 100 billions.
CAR co-express IL-12 or IL-15 can also greatly increase CAR-T killing function.
